Research on Functional Ingredients
Peptides
Research continues in multiple fields of technology ranging from reducing allergenicity to developing functional ingredients
Our aim is to protect infants from milk allergies.
Peptide research began from the development of milk for people with milk allergies.
Since 1974, we have been making effort toward the development of infant formula for those with symptoms of milk allergy. At the time our research began, food allergies and milk allergies were not well understood in Japan.
However, some infants who drink infant formula suffered from a rare type of severe non-bacterial diarrhea, called intractable diarrhea. Milk allergy was suspected to be the cause. At that time in Japan, there was no milk that could be used by infants with such severe milk allergies, and some specialists made the effort to order milk from the United States for use as treatment.

Our research team therefore felt the need to produce milk in Japan that even infants with severe milk allergies could drink without fear of adverse reactions. The team proceeded to develop a new type of milk containing a peptide that allows the infant’s digestive system to breakdown through enzymatic activity the milk protein that causes the allergy.
Allergic reactions occur when the body recognizes a specific part of a protein. It is known that when a protein is broken down and such specific part is destroyed, an allergic reaction does not occur. Milk for allergic diseases was developed with this effect in mind.
Column-1
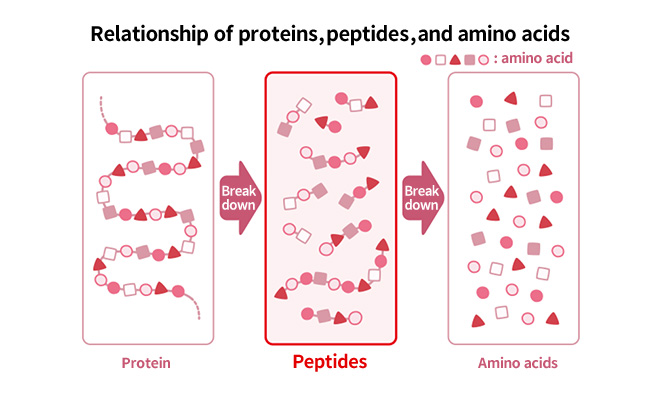
What are peptides?
- ●Peptide is a general term for substances in which about 2 to 50 amino acids are linked. When we ingest protein, it is broken down into peptides and amino acids by the action of digestive enzymes in the body. Peptides are also contained in fermented foods such as cheese and yogurt. Peptides are indeed a familiar ingredient.
- ●Peptides are characterized by their diversity. Peptides are a combination of 20 amino acids. There are 400 types (20 x 20) of peptides in combinations of two amino acids and 8,000 types (20 x 20 x 20) in combinations of three amino acids. Since peptides are known to be involved in various physiological functions as hormones in the living body, research on various bioactive peptides is being conducted all over the world while taking into account this diversity.
IgE antibodies cause allergic reactions by recognizing and binding to specific structures and amino acid arrangements of proteins. Small ones may react even with about 5 to 6 amino acid sequences. Therefore, in order to prevent allergic reactions (lowering allergens), it is necessary to reduce the molecular weight of the peptides produced by fragmentation by enzymatic digestion.
In order to reduce the allergenicity of milk protein, we conducted multiple trial production runs in which we combined all the degrading enzymes available at that time and our original lactic acid bacterium enzyme. Over time, we succeeded in developing a peptide in milk with extremely low allergenicity that hardly reacts with IgE antibodies in the patient's blood.
However, when the peptide is broken down into small pieces, the allergenicity is reduced, but the bitterness of the peptide tends to be strong. No matter how much allergenicity is reduced, there is no meaning to doing so if the infant doesn't drink the milk. A number of tests have been conducted to resolve this conundrum. Through research, it was discovered that a particular lactic acid bacterium has an enzyme that reduces bitterness, and finally the prospect of developing milk that even infants can drink came to light.
The milk MA-1 containing this hypoallergenic peptide was sold in 1977 as the first domestically produced milk for allergic conditions. This product made it possible to reduce the suffering of infants from intractable diarrhea. More than 40 years have passed since the development of MA-1. Now our current New MA-1 continues to support infants with milk allergies.
Expansion from specialty milk to infant formula
Peptide manufacturing technology which allowed the production of special milk such as MA-1 is now being used for general-purpose infant formula. Morinaga E-Akachan, which breaks down all milk proteins into small particles and takes into consideration the burden on the infant's digestive ability, has been highly evaluated by users as a infant-friendly milk.
We at Morinaga Milk hope for smiles on the faces of infants, with and without allergies, and their mothers whenever they need infant formula.
Column-2
Our unique peptide manufacturing technology and new allergenicity evaluation method
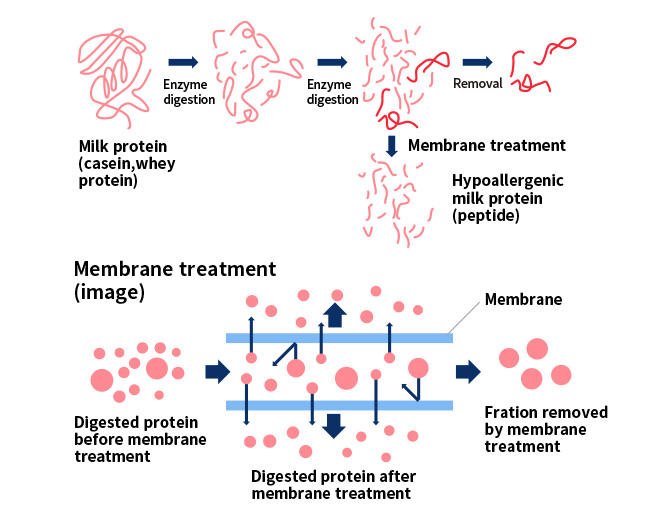
In addition to enzyme digestion, we have adopted purification technologies such as membrane separation technology * to simultaneously achieve the conflicting objectives of maintaining an appropriate flavor and reducing allergenicity. The results of this technological development support the peptide production of all milk products for allergies at Morinaga Milk.
Also, we are developing new allergenicity evaluation methods such as the Pep-iEIA method using a peptide array and the basophil activation test, and are proceeding with research to be supported by publishing a scientific paper.
* Membrane separation technology: By passing an enzyme-digested peptide through a membrane with fine pores, the peptide can be separated according to the size of the molecule (chain length).
Solving social issues Tripeptide MKP®
We are advancing the research and development of bioactive peptides that contribute to solving customers' health problems by utilizing the advanced peptide manufacturing technology cultivated in the research and development of milk for infants. One of these problems is the response to hypertension, which is expected to intensify in the future as the aging society grows larger.
Blood pressure, especially systolic blood pressure, is known to increase with age. Therefore, it can be said to be one of the most common disorders in modern society as the aging population expands. In fact, according to the World Health Organization (WHO), it is estimated that there are more than 1.2 billion people with high blood pressure in the world.
On the other hand, an increase in blood pressure is an important risk factor for many diseases such as cardiovascular diseases such as stroke and myocardial infarction, and is one of the social issues that cannot be avoided as we aim toward the realization of a healthy and happy life. Therefore, we decided to proceed with research and development of peptides with the goal of solving the growing health problem of hypertension.
It has been previously reported that some milk-derived peptides have the effect of lowering blood pressure. On the other hand, little was known about milk-derived peptides other than those already studied and reported. Questions still remained about what other kinds of peptides exist and whether those peptides have a blood pressure lowering effect. Therefore, our research team embarked on a search for other peptides using the know-how we have developed for producing peptides for products that can breakdown milk protein (i.e. milk-derived peptide compositions having various amino acid sequences). In our search, it was necessary to develop a method for efficiently measuring the activity, a method for precisely separating and purifying a composition containing a large number of peptides, and an advanced analysis technique for accurately analyzing the amino acid sequence of the isolated peptides. As a result of these efforts, we were the first to discover Tripeptide MKP
Understanding advances - functions of Tripeptide MKP

Tripeptide MKP is produced by breaking down a protein called casein found in milk with a food-related enzyme. The product name was chosen to reflect the fact that the amino acids methionine (M), lysine (K), and proline (P) are linked in order.
Tripeptide MKP inhibits the action of angiotensin converting enzyme (ACE), which produces substances that raise blood pressure. Since it was known that inhibiting the action of ACE suppresses the rise in blood pressure, it was expected that Tripeptide MKP might have a function to lower blood pressure.
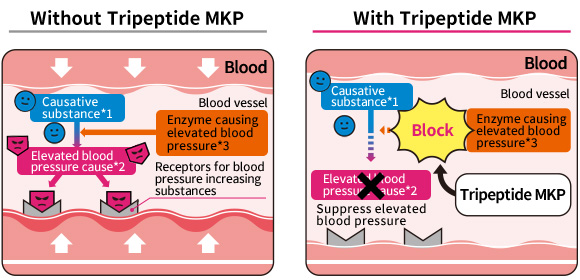
*1 Angiotensin I *2 Angiotensin II
*3 Angiotensin converting enzyme
Clinical trials and results over the years
In order to conduct clinical trials in humans, it is necessary to comply with many conditions, for example thoroughly examining safety and efficacy from multiple perspectives based on numerous scientific grounds. As a result of accumulating these scientific findings and conducting clinical tests, it was confirmed that casein enzymatic degradation products containing Tripeptide MKP lower the blood pressure (systolic blood pressure) of those people with high blood pressure.
Peptide research, which began about half a century ago with the desire to support infants, is now expanding into solving social issues faced by many people of the world. We will continue to study the potential of peptides and develop foods that take advantage of their functionality to contribute to the realization of a healthy and happy society.
