Product Development
Bifidus Yogurt
Delivering active bifidobacteria to the intestineMorinaga Milk’s breakthrough technology

Development began with research on breastmilk nutrition
Until the 1960s, the Japanese yogurt market was dominated by two main types. Those types were hard yogurt, which is sweetened and solidified with agar or gelatin, and soft yogurt, which is made into a smooth semi-solid yogurt with added fruit pulp. Both had a sweet taste and were eaten as snacks and desserts. At the Japan World Exposition (Expo ’70) held in Osaka, Japan, plain yogurt without added sugar was introduced to the Japanese people.
While conducting research and development of infant formula, we studied the differences between these products and breast milk nutrition focusing on intestinal flora. In particular, we looked at bifidobacteria. Utilizing the technology and knowledge of bifidobacteria cultivated in that research, we started our efforts to develop yogurt containing bifidobacteria.
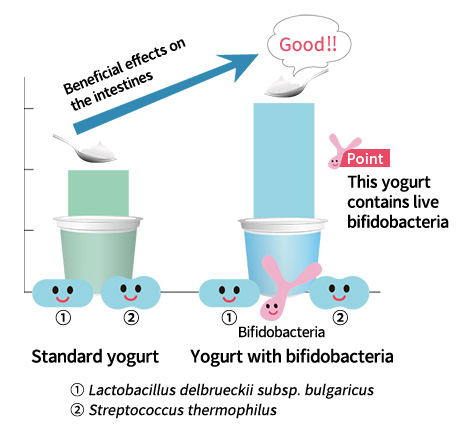
Although many people think that all yogurt contains bifidobacteria, this is not necessarily the case. Bifidobacteria are very different from the lactic acid bacteria used in generally available yogurt, and are very difficult to handle because of their sensitivity to acid and oxygen.
In 1971, we succeeded in the commercial production of fermented milk containing bifidobacteria after overcoming the various technical issues of adding bifidobacteria to yogurt. After that, further improvements were made, and our Bifidus Yogurt was released into the yogurt market in 1978.

Left: Bifidus Yogurt at the time of launch
Right: Bifidus Yogurt currently on sale (as of March 2022)
In order to offer live bifidobacteria to customers, it is necessary to overcome certain properties of bifidobacteria, such as their inability to withstand exposure to oxygen and their inability to remain viable in yogurt. For this reason, we made improvements in the manufacturing method of bifidobacteria starter and developed a container with low oxygen permeability to stabilize the viability of the bifidobacteria. These developments allowed us to manufacture the product we had envisioned. In recognition of the results of our research, Bifidus Yogurt was approved as a Food for Specified Health Uses in 1996.
Development of breakthrough technology to deliver live bifidobacteria to the intestine
On the market today there are many yogurt products that depend only on lactic acid bacteria. On the other hand, Bifidus Yogurt contains both lactic acid bacteria and bifidobacteria. Bifidobacteria are generally weak when exposed to acid and oxygen so not all of the bifidobacteria can multiply in yogurt. In addition, even if they can proliferate during fermentation, many bifidobacteria die before the product expiration date, making it difficult to provide yogurt with abundant live bifidobacteria. Therefore, in order to ensure that the bifidobacteria will reach the intestine is a viable state, we developed a unique technology that protects the bacteria from acid and oxygen. This technology was epoch-making at the time, and in 1984 Morinaga Milk received the Science and Technology Agency Director-General's Award for our efforts in developing this technology.
Bifidobacterium longum BB536
– expected to have a wide range of functions
Bifidobacterium longum BB536 in Bifidus Yogurt is a type of Bifidobacterium found in the intestine of infants. This bacterium is added to foods not only in Japan but also in over 30 countries around the world to support human health. Many of the products containing bifidobacteria currently on the market use animal residential bifidobacteria, but we believe that the most suitable for humans is the human residential bifidobacteria. More than 200 research papers have been published on Bifidobacterium longum BB536 to date. Research continues on the various functionalities of bifidobacteria including intestinal regulation.
One more step for yogurt – research on functional foods
Morinaga Milk is also developing and selling various functional foods in the Bifidus Yogurt series. In particular, the production methods of Foods for Specified Health Uses (FOSHU) and foods with function claims require strict control of the number of bifidobacteria in the products. It is critical that we maintain the number of viable bifidobacteria in each product through the expiration date. Because the manufacturing plant produces large amounts per batch, the results of production at the plant may differ from that of the laboratory. Such results may also be affected by the raw materials, manufacturing processes, product size, shape, packaging, etc., so we constantly examine the product formulation and manufacturing conditions and develop new technologies as necessary. The Bifidus Yogurt series is achieving its mission to provide living bifidobacteria in a consistent manner along with offering new and different yogurt products to consumers.
